CRISPR gene editing has revolutionized the field of genetics, offering unprecedented opportunities to modify organisms at the DNA level. This powerful technology enables scientists to target and edit genes with remarkable precision, potentially leading to cures for debilitating conditions like sickle cell disease. However, the promise of such advancements raises critical ethical questions about genetic modification, particularly concerning health equity and access to these groundbreaking treatments. As researchers explore the implications of CRISPR technology, the global community must navigate the delicate balance between innovation and ethical responsibility in gene editing. Ultimately, the discussions surrounding ethical gene editing will shape the future of medicine as we learn to harness this transformative tool for the betterment of humanity.
Gene editing through advanced methodologies such as CRISPR has sparked a wave of interest and debate within the scientific community and beyond. This innovative approach enables precise alterations to genetic material, unlocking the potential for novel treatments and even cures for various genetic disorders. While the scientific advancements present promising solutions, they also prompt inquiries into the morality and implications of altering human genetics. Discussions around genetic modification ethics are essential, particularly as we consider the social ramifications of health equity in gene editing. As we delve into the realm of this cutting-edge technology, it’s imperative to address the underlying questions about its use and the responsibilities that come along with such breakthroughs.
Exploring the Ethics of CRISPR Gene Editing
The astonishing advancements in CRISPR gene editing technology have opened the door to potentially eradicate genetic conditions like sickle cell anemia. However, these breakthroughs have ignited fierce debates surrounding the ethics of such interventions. With the ability to modify the very blueprint of life, society faces critical questions: Should we intervene in the natural genetic diversity that defines humanity? As we navigate the ethical landscape of gene editing, it is crucial to weigh the benefits of advanced cures against the potential for misuse or unintended consequences. Prominent figures in the field urge the importance of implementing strict guidelines that align technological advancements with moral responsibilities.
In discussions led by experts like Neal Baer, the implications of gene editing extend beyond individual cases. The ethics of choosing traits and preferences—such as the decision of whether to allow parents to opt for a hearing child over a deaf one—raise fundamental questions about autonomy and societal values. These dilemmas highlight the necessity for robust ethical frameworks as CRISPR technology becomes more accessible. The intersection of medical potential and ethical concerns presents a challenging yet critical dialogue that society must address.
The Promise of Curing Sickle Cell Disease
Curing sickle cell disease through CRISPR technology represents a significant milestone in medical science. With around 100,000 individuals affected in the United States, the potential to alleviate suffering is immense. The ability to edit faulty genes that lead to this painful condition could not only enhance the quality of life for those impacted but also reduce the emotional and financial burdens borne by families. The treatment, however, comes with a hefty price tag—approximately $2.2 million—which sparks an urgent conversation about health equity in gene editing.
Beyond the immediate promise of a cure, there are broader implications to consider. As the costs of gene therapies remain high, accessing these innovative treatments may be beyond the reach of many, leading to systemic inequalities in healthcare. Discussions about who can afford such groundbreaking treatments raise essential questions about fairness in the distribution of medical resources. The advancements in CRISPR gene editing must not only focus on technical success but also address the equitable availability of such life-changing therapies to ensure that all members of society can benefit.
Health Equity in Gene Editing
As CRISPR gene editing technology advances, the issue of health equity becomes increasingly salient. The promise of these medical innovations cannot be fully realized unless access is equitable among diverse populations. The exorbitant costs associated with gene therapies like those for sickle cell disease must be reconciled with the reality that many individuals worldwide remain excluded from these advancements. Without careful consideration of the socioeconomic factors influencing access to CRISPR treatments, we risk exacerbating existing disparities within healthcare systems.
Public discourse must therefore focus not only on the miraculous potential of gene editing but also on assuring that these life-saving technologies reach marginalized communities. Advocates argue for the necessity of establishing policies and frameworks that prioritize equitable access to healthcare innovations, thereby promoting a fair distribution of resources across all demographics. Addressing these disparities will help fulfill the promise of CRISPR technology as a tool for health equity and social justice.
The Future of Genetic Modification Ethics
The landscape of genetic modification is rapidly evolving with transformative technologies like CRISPR, but with great innovation comes significant ethical concerns. As scientists unlock the capabilities to alter genetic material, critical discussions arise about the boundaries of what should or should not be modified. This ethical discourse encompasses not only the medical and biological implications but also the societal and philosophical ramifications of choosing specific traits or conditions. The conversation should include a wide range of stakeholders—including scientists, ethicists, and the public—to cultivate a comprehensive understanding of these issues.
As the dialogue evolves around the ethics of gene editing, it is incumbent upon society to establish a moral framework that guides the use of CRISPR technology. Future advancements must tread carefully to ensure that the pursuit of progress does not lead us down a path of genetic inequality or unintended consequences. The long-term goal should be to facilitate responsible innovation that enhances human health without sacrificing ethical standards. Only through collaborative efforts can we navigate the intricacies of genetic modification ethics and find a balanced approach that respects diversity and human dignity.
The Role of Oversight in Gene Editing
With the advent of CRISPR gene editing, establishing effective oversight mechanisms is more crucial than ever. While the technology holds incredible potential for advancing human health, the possibility of misuse, particularly in unregulated environments, poses significant risks. Historical examples of genetic experimentation in various countries underscore the pressing need for international regulations that ensure the ethical application of these powerful tools. As gene editing technologies expand, oversight bodies must be vigilant to address the ethical dilemmas arising from genetic experimentation.
Furthermore, transparency in the governance of genetic editing research will play a vital role in gaining public trust. Discussions led by experts like Neal Baer highlight the importance of regulatory frameworks that balance innovation with safety. Ensuring strict oversight can help prevent unethical practices, such as the genetic modification of embryos without consent or the enhancement of physical or cognitive traits that reinforce societal inequalities. A commitment to ethical oversight is essential for guiding the responsible use of CRISPR technology and safeguarding against potential abuses.
Navigating Unintended Consequences of Gene Editing
One of the primary challenges associated with CRISPR gene editing is the possibility of unintended consequences. While successful edits may address specific genetic disorders, such interventions can disrupt complex genetic interactions, leading to unforeseen health issues. For example, altering genes involved in cholesterol levels may affect a cascade of other genetic processes, raising concerns about collateral damage. Researchers are tasked with understanding the intricate web of gene interactions before liberally applying CRISPR technology to human health.
It is imperative that the scientific community remains vigilant in studying the long-term effects of gene modifications. Continuous monitoring and research into the aftermath of genetic editing are necessary to safeguard patients from potential harm. The goal is not simply creating cures but ensuring that those cures come without additional risks. As CRISPR continues to revolutionize the medical landscape, ethical research practices must prioritize comprehensive evaluations of outcomes to mitigate the risks associated with unintended consequences.
The Intersection of Technology and Human Variation
In the discourse surrounding CRISPR and gene editing, it is essential to acknowledge the diversity of human variation. As Baer points out, attributes like deafness should not always be viewed through the lens of pathology; rather, they can represent aspects of cultural identity. The capability to alter these traits raises philosophical questions about the extent to which we should exert control over our genetic heritage. Societal perceptions of ‘normal’ versus ‘abnormal’ significantly influence decisions about gene editing, and it is vital to confront these biases as we navigate the future of genetic modification.
As discussions unfold, embracing the concept of human variation as a natural part of diversity can shift our understanding of genetic modifications. The conversation should encompass the respect and appreciation for all forms of human expression rather than strictly aiming for conformity to societal norms. By fostering an environment of acceptance, we can create a more nuanced perspective on the potential applications of CRISPR technology, recognizing that not all genetic differences necessarily need ‘correcting’ but may contribute positively to the rich tapestry of human experience.
Cultivating Informed Public Engagement on Gene Editing
As advancements in CRISPR technology reshape the medical landscape, fostering informed public engagement becomes imperative. The general population must be equipped with knowledge about the implications of gene editing—both positive and negative. Educational initiatives can help demystify the science behind CRISPR while promoting discussions about its ethical dimensions. Engaging communities in dialogues surrounding genetic modification not only empowers individuals to voice their opinions but also reinforces the importance of democratic decision-making in scientific advancements.
Through public forums, workshops, and informative media, citizens can explore the societal implications of gene editing. By highlighting diverse perspectives, stakeholder engagement encourages a balanced approach that incorporates the views of those who may be directly affected by these technologies. Informed and inclusive discussions set the stage for establishing guidelines that reflect societal values while allowing for innovation. Cultivating public understanding is key to ensuring that the developments in CRISPR technology align with the needs and ethical considerations of the broader community.
Frequently Asked Questions
What is CRISPR gene editing and how does it work?
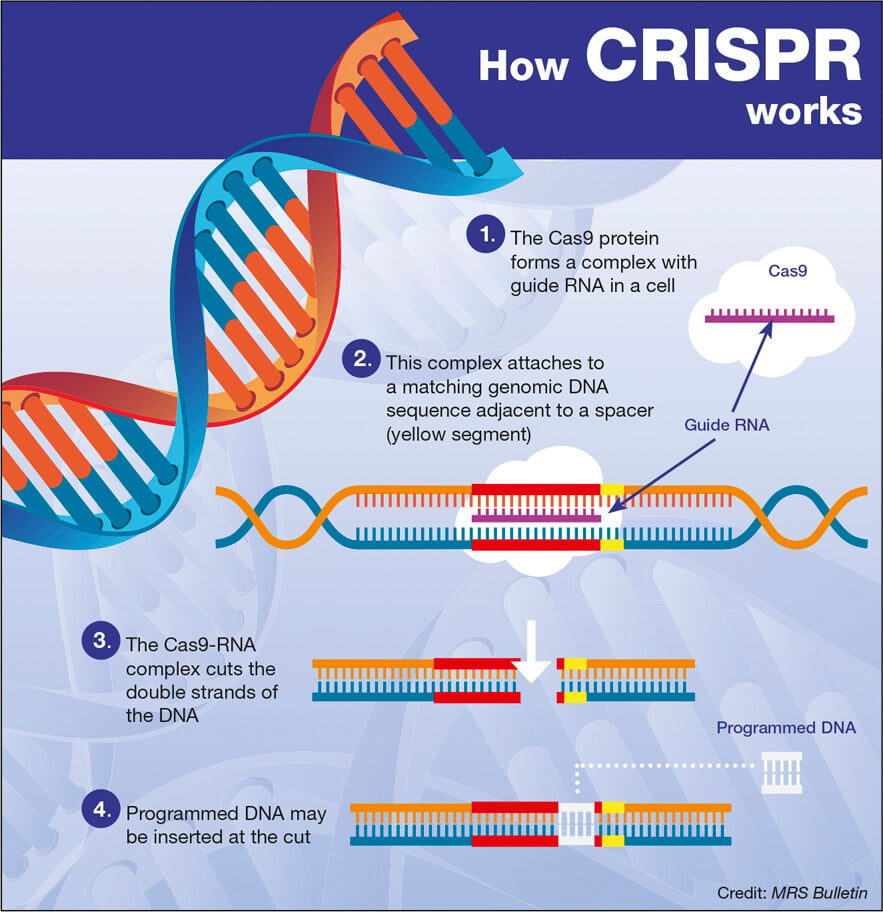
CRISPR gene editing is a revolutionary technology that allows scientists to modify an organism’s DNA with precision. By utilizing a guide RNA to identify specific DNA sequences, CRISPR technology can cut the DNA at the target site, enabling the removal or replacement of defective genes. This innovative approach has significant implications for treating genetic disorders, such as sickle cell disease.
What are the ethical implications of CRISPR gene editing?
The ethical implications of CRISPR gene editing are vast and complex. Prominent issues include the potential for ‘designer babies,’ where genetic modifications are made for non-medical traits, and the fairness of access to these technologies, particularly in cases like sickle cell cure. Discussions on ethical gene editing continue to explore the responsibility of scientists and society regarding genetic modifications and health equity.
Can CRISPR technology cure sickle cell disease?
Yes, CRISPR technology has shown promise in curing sickle cell disease by editing the genes responsible for the disorder. Researchers can manipulate the somatic cells to correct the defective gene, potentially providing a lifelong cure. However, the high costs and ethical debates surrounding access to this treatment raise significant concerns in the realm of health equity in gene editing.
What are the health equity concerns related to CRISPR gene editing?
Health equity concerns in CRISPR gene editing involve the disparity in access to these advanced treatments. For instance, although CRISPR can cure sickle cell disease, the associated costs of around $2.2 million create barriers for many patients. Therefore, discussions around health equity focus on ensuring that all populations, regardless of socioeconomic status, have fair access to the benefits of CRISPR technology.
Who decides the ethical boundaries of genetic modification using CRISPR technology?
Decisions regarding the ethical boundaries of genetic modification using CRISPR technology are made by a combination of scientists, ethicists, policymakers, and society at large. Engaging in public discourse, addressing ethical gene editing concerns, and creating regulatory frameworks are crucial steps in determining who will oversee and decide the limits of genetic alterations.
Are there risks associated with CRISPR gene editing?
Yes, CRISPR gene editing carries several risks, including potential unintended genetic consequences and off-target effects where genes not intended for editing may be altered. As genes interact in complex ways, unintended modifications could lead to unforeseen health issues. Ongoing studies aim to refine CRISPR technology to minimize these risks and ensure its safe application in gene editing.
How does CRISPR gene editing challenge traditional views of human variation?
CRISPR gene editing challenges traditional views of human variation by introducing the ability to edit traits that are often considered natural variations. This raises ethical questions about whether certain conditions, like deafness, should be seen as something that needs ‘fixing.’ Critics argue that not all variations constitute a pathology, emphasizing the need for sensitivity in discussions about genetic modification.
What precautions exist to regulate CRISPR and genetic editing globally?
Global regulations for CRISPR and genetic editing vary by country. While certain nations have strict laws against germline editing, others may lack enforcement or oversight. The need for international cooperation and ethical guidelines is essential to ensure that ethical gene editing practices are upheld and to prevent misuse, particularly in countries less restrained by such regulations.
| Key Point | Details |
|---|---|
| CRISPR Technology Basics | CRISPR allows for precise edits to DNA, targeting both somatic and germline genes. |
| Ethics of Gene Editing | Questions arise about the morality of altering traits in humans and who decides these changes. |
| Cost of Treatments | CRISPR treatments, like that for sickle cell, can exceed $2.2 million, raising issues of access. |
| Health Equity | The disparity in access to gene editing technology highlights the challenge of health justice. |
| Potential for Unintended Consequences | Altering genes could result in unforeseen health issues due to complex gene interactions. |
| Regulatory Oversight | Current laws, like those against cloning, may not be effectively enforced worldwide. |
Summary
CRISPR gene editing represents a groundbreaking advancement in biotechnology, offering the potential to cure genetic diseases and alter human traits. However, as highlighted in recent discussions, it raises significant ethical dilemmas regarding its application. The balance between innovation and health equity is essential, as societal disparities in access to these technologies can exacerbate existing injustices. Moreover, the prospect of unintended consequences and the need for stringent regulatory oversight underscore the complex landscape we navigate with CRISPR. As we continue to explore these frontiers, it is vital to engage in thoughtful dialogue about the implications of gene editing on humanity.