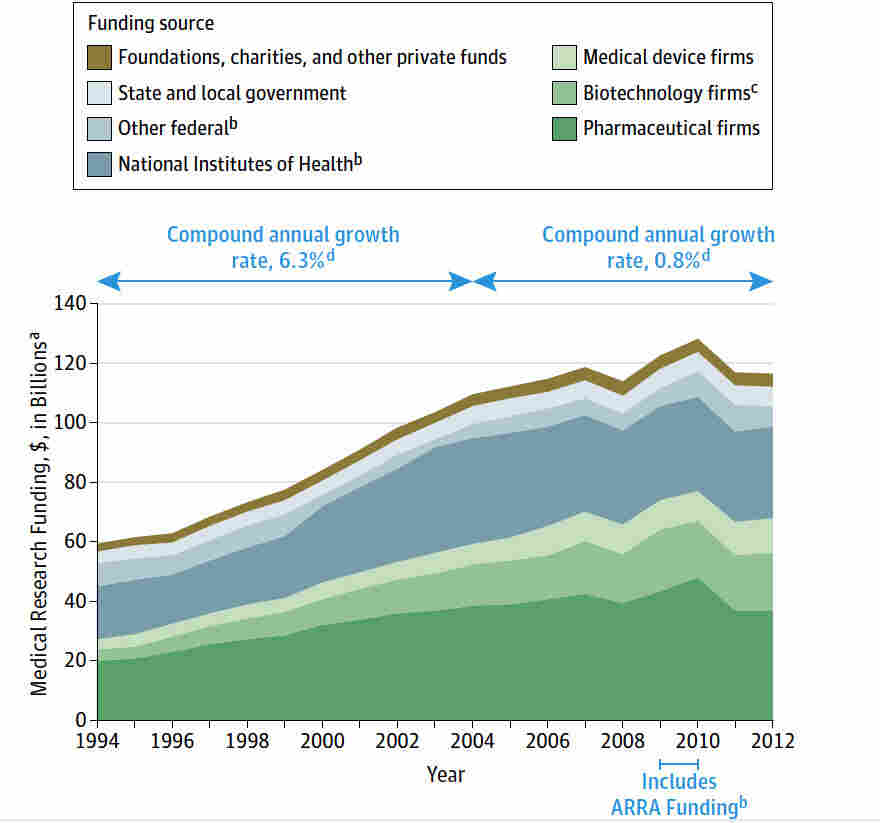
Medical research funding plays a pivotal role in advancing healthcare and ensuring patient safety during studies. As institutions like the National Institutes of Health (NIH) allocate essential resources for research, they oversee compliance with research ethics and institutional review board (IRB) requirements. Adequate funding allows clinical trials to be conducted efficiently, guaranteeing that the rights of participants are protected under strict oversight. However, recent funding cuts threaten to disrupt these vital processes, jeopardizing patient safety and the integrity of innovative studies. Understanding the implications of reduced financial support is crucial as it directly influences the outcomes of medical research that can save lives.
In the realm of healthcare innovation, the provision of financial resources for scientific inquiry is crucial in safeguarding the welfare of individuals participating in clinical evaluations. When discussing fiscal allocations for health-related studies, terms such as research grants, NIH support, and ethics oversight come into play, shedding light on the systemic challenges faced by medical researchers. The oversight provided by committees monitoring ethical practices, particularly the institutional review boards, is crucial in maintaining a standard for patient involvement in therapeutic investigations. Moreover, the effectiveness of these clinical trials largely hinges on adequate funding to ensure comprehensive support for ethical guidelines and participant consent processes. The intersection of funding, patient welfare, and research integrity necessitates a holistic view for those involved in health advancements.
The Impact of NIH Funding on Patient Safety in Research
NIH funding plays a crucial role in enhancing patient safety within medical research trials. With consistent financial support from organizations like the National Institutes of Health, research institutions can establish robust protocols and oversight mechanisms through Institutional Review Boards (IRBs). These boards ensure that all research involving human participants adheres to stringent ethical guidelines, safeguarding their rights and well-being throughout the study. By maintaining rigorous standards and accountability, NIH funding helps to create a safer environment for trial participants, ultimately leading to more reliable and ethical research outcomes.
Moreover, NIH funding covers costs associated with training personnel, conducting compliance audits, and implementing risk mitigation strategies to protect patients during clinical trials. Without adequate financial resources, many research projects may struggle to meet the necessary ethical standards mandated by IRBs, increasing the risk of compromising participant safety. The emphasis on patient welfare in NIH-funded projects not only boosts public trust in medical research but also encourages broader participation, thereby advancing scientific discovery and innovation.
The Role of IRBs in Medical Research Oversight
Institutional Review Boards (IRBs) serve as the backbone of ethical oversight in medical research, acting as a critical gatekeeper for the protection of human subjects. Their responsibilities encompass comprehensive reviews of research proposals, where they assess the potential risks and benefits, the informed consent process, and the overall ethical implications of the study. The proactive involvement of IRBs ensures that participants are well-informed and safeguarded against potential harm, reflecting a commitment to research ethics that is integral to the integrity of clinical trials.
Additionally, IRBs provide education and support to researchers, fostering an environment where ethical considerations are paramount. They work closely with investigators to develop protocols that align with federal regulations and ethical standards, thus enhancing patient safety throughout the research lifecycle. As clinical trials evolve and new challenges emerge, the role of IRBs becomes even more critical, ensuring that researchers maintain a focus on participant welfare, which ultimately strengthens the foundation of trustworthy medical advancements.
How Funding Cuts Impact Research Ethics and Patient Care
Recent funding cuts, particularly in federal grants, pose a significant threat to the integrity of medical research and patient care. The cessation of financial support for critical initiatives such as SMART IRB disrupts the essential oversight that protects research participants. Many studies face halts mid-progress, leading to ethical dilemmas where patients may be left vulnerable without the necessary oversight mechanisms in place. As research projects stagnate, the public’s trust in medical institutions and the research process can erode, further complicating efforts to recruit participants for future trials.
Moreover, the absence of adequate resources for IRBs and research teams can lead to insufficient training and support for investigators. This lack of preparation raises the likelihood of ethical violations, potentially compromising patient safety and adherence to research ethics standards. When financial backing is reliable, institutions can prioritize patient well-being, ensuring thorough IRB reviews that mitigate risks effectively. The cascading effects of funding cuts not only endanger the participants involved but could also threaten the aspirations of medical advancements that benefit society at large.
Collaborative Research and Patient Safety Risks
Collaborative research presents a unique set of challenges and opportunities regarding patient safety. Effective coordination between multiple sites enhances the potential for significant medical breakthroughs; however, each site must still comply with rigorous intervention protocols aligned with research ethics. The implementation of a single IRB model has streamlined processes for multisite studies, enabling faster approvals and bolstering comprehensive oversight for patient safety. This seamless approval process is often supported by federal funding, which underpins the collaboration among institutions and ensures consistent ethical standards are met.
However, disruptions in funding flow, as seen recently, have the potential to derail collaborative efforts, leading to delays in patient recruitment and the onset of behind-schedule treatments. As research protocols come into question due to a lack of resources, ethical oversight may weaken, elevating the risks faced by participants. Collaborative research should thrive on the integrity of its structure; without it, patient safety cannot be guaranteed, and the reputation of clinical research as a whole suffers, further complicating future collaborations.
The Historical Context of Research Ethics
Understanding the historical context of medical research ethics provides crucial insight into present-day practices aimed at ensuring patient safety. Landmark events such as the Tuskegee Syphilis Study and the atrocities of World War II have catalyzed a paradigm shift toward stringent ethical oversight in human research. These events raised critical awareness about the need for oversight mechanisms such as IRBs, which are designed to protect participants from exploitation and harm. As a result, adherence to ethical standards has become non-negotiable in the research process.
This historical backdrop emphasizes the monumental importance of continuous funding for research projects aimed at protecting human subjects. Adequate financial resources enable institutions to uphold these ethical standards through rigorous training and compliance monitoring, ensuring that all research activities prioritize the welfare of participants. The lessons learned from past injustices serve as a guiding framework for current medical research practices, underscoring the vital need for research ethics and protection mechanisms that prioritize patient safety.
Communicating Risks to Participants in Clinical Trials
Effective communication of risks to research participants is a cornerstone of ethical medical research. Ensuring that potential participants fully understand the possible risks and benefits supports informed consent and fosters trust between researchers and participants. IRBs play an essential role in reviewing communication materials and ensuring that all information provided to participants is accessible, accurate, and comprehensive. This commitment to transparency is vital, as it empowers participants to make informed choices about their involvement in clinical trials.
In recent discussions on research ethics, funding for regulatory frameworks that support effective communication practices has garnered increasing attention. When financial resources are provisioned for training and development in participant engagement, researchers can enhance their approaches to informing subjects about risks effectively. Furthermore, fostering an open dialogue between the research team and participants encourages ongoing discussions, allowing participants to voice concerns and ask questions throughout the trial. This two-way communication strategy is essential in maintaining patient safety and establishing a culture of mutual respect and transparency in clinical research.
Future Directions for Medical Research Funding
As medical research continues to evolve, the future landscape of funding must adapt to meet emerging challenges and opportunities. Ensuring robust funding for patient safety initiatives and research ethics programs is paramount to maintaining the integrity of medical research. Stakeholders, including federal agencies and academic institutions, must prioritize investment in innovative funding strategies that support ethical oversight, particularly for collaborative and multisite studies that require additional resources to maintain patient safety.
Moreover, the funding landscape should reflect a commitment to fostering diverse research initiatives that consider varying patient populations and their unique needs. By broadening the focus of medical research funding to encompass various aspects of patient safety and ethics, we encourage an inclusive approach that prioritizes the well-being of all participants. The future of medical research funding must integrate these priorities to build systems that can withstand challenges and ensure ethical standards persist, ultimately preserving trust in the research process.
The Interplay of Ethics and Innovation in Medical Trials
The relationship between ethics and innovation in medical research is a delicate balance that has significant implications for patient safety. Ethical principles serve as the foundation upon which innovative research practices are built; a lack of ethical consideration can undermine the validity and safety of clinical trials. As researchers strive to push the boundaries of scientific discovery, they must simultaneously navigate ethical responsibilities that ensure participant protection. Funding mechanisms that prioritize both ethical oversight and innovation are crucial to advancing medical research while safeguarding the rights and welfare of participants.
In order to promote innovation within a narrow ethical framework, researchers must engage in continuous dialogue with ethical committees and IRBs to align their goals with patient safety standards. This collaborative approach encourages a culture where ethical considerations inform innovative practices, ultimately leading to responsible breakthroughs in medical research. Ensuring sufficient funding for both research and ethical oversight initiatives contributes to a thriving research environment that successfully marries creativity and patient safety.
Strengthening Community Engagement in Research
Community engagement plays an essential role in fostering trust between researchers and participants, particularly in clinical trials. By actively involving community members in the research process, institutions can better understand the concerns and values of potential participants. This participatory approach not only enhances recruitment efforts but also empowers individuals to advocate for their safety within research settings. When funded adequately, community outreach initiatives can facilitate ongoing dialogue, ensuring that research practices remain transparent and ethical.
Furthermore, as research funding becomes more limited, prioritizing community engagement becomes even more critical. Robust funding for engagement programs aids researchers in developing strategies that address the specific needs of diverse populations, enhancing the relevance and effectiveness of clinical trials. Engaging communities fosters a sense of ownership and accountability in research, thereby promoting ethical practices that prioritize patient safety. Ultimately, strengthening community involvement ensures that research reflects the values and interests of the populations it seeks to serve.
Frequently Asked Questions
How does NIH funding contribute to patient safety in medical research?
NIH funding plays a crucial role in ensuring patient safety in medical research by supporting the Institutional Review Board (IRB) oversight that monitors research involving human participants. This funding allows for thorough reviews of research proposals to safeguard the rights and welfare of participants, ensuring that ethical standards are upheld throughout the research process.
What is the impact of IRB oversight on clinical trials funded by the NIH?
IRB oversight is essential for clinical trials, particularly those funded by the NIH. It ensures that studies comply with ethical guidelines, protect patient rights, and monitor safety throughout the research lifecycle. By providing a layer of scrutiny, IRBs help mitigate risks associated with clinical trials, enhancing the integrity of the research conducted.
How do funding cuts affect research ethics in medical studies?
Funding cuts significantly impair research ethics in medical studies by limiting the ability of IRBs to operate effectively. Reduced NIH funding can lead to fewer resources dedicated to oversight, potentially compromising the ethical review process and the protection of patients involved in research, which could diminish trust in the research community.
What are the consequences of halted medical research funding on patient safety?
Halting research funding can have dire consequences for patient safety by disrupting ongoing studies and delaying crucial trials that aim to improve treatments. This can lead to gap periods where patient safety is compromised due to a lack of oversight and monitoring, potentially resulting in adverse outcomes for participants.
Why is NIH funding essential for collaborative research and its impact on patient safety?
NIH funding is essential for collaborative research as it supports the establishment of systems like SMART IRB, which streamline the oversight of multi-site studies. This collaborative approach enhances patient safety by ensuring that all participating sites adhere to unified ethical standards, thereby protecting the well-being of all study participants across locations.
How does patient safety influence public trust in medical research funding?
Patient safety is a vital component that directly influences public trust in medical research funding. When research is conducted ethically and with robust oversight, funded by organizations like the NIH, it fosters greater confidence among the public. Conversely, funding cuts can undermine this trust by leading to perceived lapses in safety and ethical standards.
What steps can be taken to improve funding for research ethics and patient safety?
To improve funding for research ethics and patient safety, advocacy for increased NIH funding is critical. Engaging with lawmakers and stakeholders to highlight the importance of ethical oversight, supporting initiatives that foster collaboration between research institutions, and raising public awareness about the implications of funding on patient safety can all contribute to securing necessary resources.
How does the SMART IRB contribute to effective patient safety in multi-site clinical trials?
The SMART IRB program enhances patient safety in multi-site clinical trials by establishing a single IRB review system, which simplifies the approval process and ensures consistent oversight across all participating sites. This approach minimizes delays in trials and maintains high standards of patient protection throughout the research.
| Key Points | Details |
|---|---|
| Funding Freeze | The Trump administration froze over $2 billion in federal research grants to Harvard, disrupting patient safety and rights in medical research. |
| SMART IRB Overview | SMART IRB is a system administered by Harvard Catalyst to facilitate oversight for research conducted at multiple sites. |
| Role of IRBs | Institutional Review Boards (IRBs) review and oversee research proposals to ensure patient safety and compliance with laws. |
| Impact on Research | Funding cuts hinder ongoing studies and add barriers to collaboration, risking participant safety and trust in research. |
| Historical Context | Past ethical breaches in research have shaped oversight systems like the IRB, ensuring participant safety in trials. |
| Future of Research | Funding cuts could stunt scientific progress and innovation, impacting treatment developments for diseases like Alzheimer’s. |
Summary
Medical research funding is crucial for ensuring patient safety and ethical oversight in clinical trials. The recent halt in funding poses a significant threat to the integrity of research processes and the welfare of participants involved. With cuts affecting organizations like SMART IRB, the risk of participant harm increases, and public trust in medical research may wane. Sustaining adequate funding is essential to uphold the ethics of medical research and to support the health and safety of potential study participants.