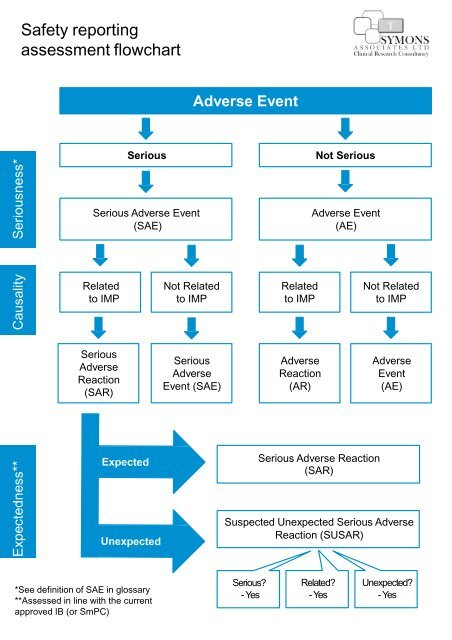
Medical research safety is paramount in ensuring the ethical treatment and protection of individuals participating in clinical studies. With the recent halt in federal research funding, vital oversight mechanisms, including the work of Institutional Review Boards (IRBs), are at risk. These boards are responsible for maintaining patient safety in research, evaluating the potential risks and benefits, and ensuring compliance with ethical standards. Yet, as funding cuts threaten the stability of research initiatives, concerns arise regarding the ability to uphold clinical trial ethics and safeguard patient welfare. Such challenges emphasize the importance of continued support for IRB oversight and federal research funding, such as those provided by the NIH, to foster a safe environment for participants involved in medical research.
Ensuring the safety of individuals involved in medical experimentation is crucial for preserving trust and ethical conduct in health-related research. The integrity of research practices hinges on effective regulatory oversight, often facilitated by dedicated committees known as Institutional Review Boards (IRBs). These entities play a vital role in evaluating study protocols and protecting the rights of participants. As we grapple with the consequences of funding interruptions, the dialogue surrounding patient welfare and ethical oversight in clinical research becomes increasingly relevant. Therefore, fostering a culture of safety and transparency is essential for advancing scientific knowledge and improving community health outcomes.
Impacts of Federal Funding Cuts on Patient Safety in Medical Research
The recent halt in federal research funding has profound implications for patient safety within medical research. When financial support is cut off, research institutions struggle to maintain the level of oversight necessary to protect participants. With over $2 billion in NIH funding affected, organizations like SMART IRB face severe operational challenges. As a result, studies that focus on ensuring participant rights and safety can be disrupted, raising concerns about the ethical conduct of research. Without adequate funding, oversight can diminish, leading to potential risks for those involved in clinical trials.
Moreover, the cuts in funding could hinder the collaborative nature of research essential for developing new treatments. When the autonomy of institutions is compromised due to lack of resources, it often translates into a decreased capacity to adhere to safety protocols. Participants may find themselves in studies that lack rigorous oversight, ultimately affecting their welfare. The repercussions extend beyond individual studies, as public sentiment and trust in the medical research process can be adversely impacted. This decline in trust makes individuals less likely to participate in clinical trials, further stymying advancement in medical science.
Frequently Asked Questions
What is the significance of IRB oversight in ensuring patient safety in medical research?
IRB oversight is crucial in ensuring patient safety in medical research. Institutional Review Boards (IRBs) are responsible for reviewing research proposals to ensure that patient rights and welfare are protected. This includes assessing study risks, obtaining informed consent, and monitoring adverse events. Without IRB oversight, research could inadvertently harm participants, undermining trust in the research process.
How do funding cuts impact patient safety in medical research?
Funding cuts significantly impact patient safety in medical research as they can halt ongoing studies and disrupt IRB oversight. This disruption can prevent new sites from opening and delay critical studies, putting participants at risk and eroding public trust in the research system. Investment in federal research funding is vital for maintaining the ethical standards that protect patients involved in studies.
What role do federal research funds play in strengthening patient safety measures?
Federal research funds, such as those from NIH, are essential for supporting the infrastructure that ensures patient safety in medical research. These funds facilitate the operation of IRBs, the training of research personnel, and compliance with ethical standards. As a result, they help create a rigorous oversight environment that prioritizes the safety and rights of research participants.
Why is patient safety a key focus in clinical trial ethics?
Patient safety is a key focus in clinical trial ethics because protecting participants is fundamental to the integrity of research. Ethical guidelines dictate that participants must be informed of risks, benefits, and burdens associated with their involvement. Prioritizing patient safety helps ensure that research is conducted responsibly, fostering public trust and promoting ongoing participation in clinical trials.
How does NIH funding impact patient safety in medical research?
NIH funding directly impacts patient safety in medical research by providing resources for rigorous oversight through IRBs. This funding supports the operational costs of conducting ethical reviews, which are vital for safeguarding participants’ rights and well-being. A strong funding base allows for continued advancements in research while maintaining high standards of patient care.
How do historical events shape current medical research safety practices?
Historical events such as the Tuskegee Syphilis Study have profoundly shaped current medical research safety practices. These events led to the establishment of ethical guidelines and IRB oversight, emphasizing the importance of informed consent and participant protection. Lessons learned from these tragedies continue to drive the commitment to patient safety in contemporary research protocols.
| Key Points |
|---|
| The Trump administration frozen federal research grants impacting safety in medical research, particularly patient protections. |
| The stop-work order disrupts SMART IRB, which facilitates oversight for multiple-site studies and ensures ethical patient treatment. |
| IRBs are fundamental for overseeing research, ensuring compliance with ethical standards, and protecting human subjects in studies. |
| Funding cuts severely impact research progress and can lead to increased risks for participants due to halted studies and disrupted processes. |
| Historically significant events have led to the establishment of strict HRPPs and IRBs which protect human subjects in research. |
| The continuation of federal research funding is critical to the safety, trust, and ethical oversight of the medical research enterprise. |
Summary
Medical research safety is crucial for protecting patients involved in studies. The recent funding cuts have highlighted the importance of federal support in maintaining ethical standards and oversight. Without adequate funding for independent review boards, the safety and rights of patients in medical trials could be at significant risk, ultimately undermining public trust in medical research. Ensuring that these safeguards are upheld is essential not only for current studies but also for advancing future innovations in healthcare.