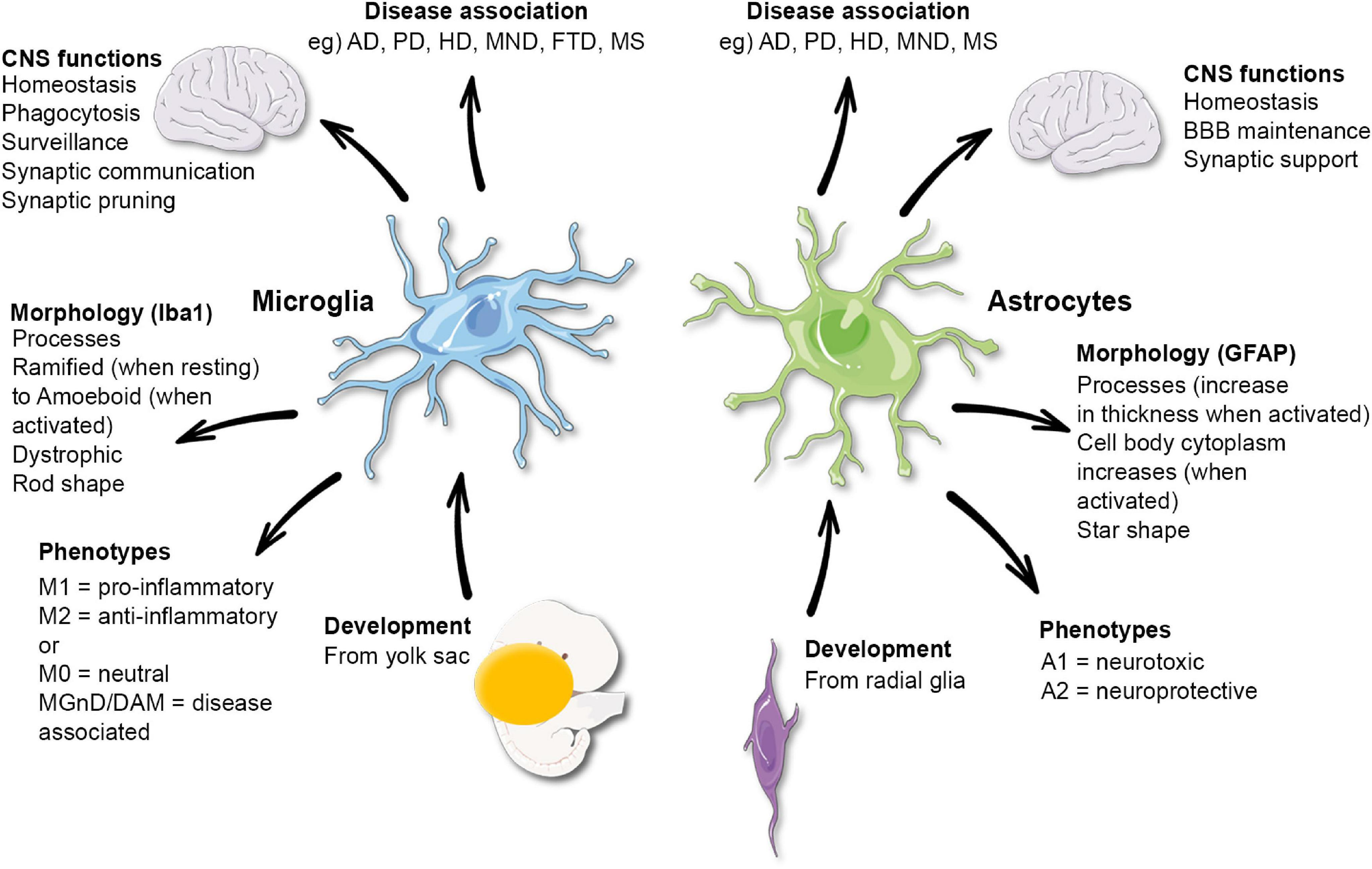
Microglial research is at the forefront of neuroscience, especially in the realm of neurodegenerative diseases such as Alzheimer’s disease. These specialized cells serve as the brain’s primary immune defense, constantly monitoring for threats like inflammation and cellular debris. Led by pioneering neuroscientist Beth Stevens, significant strides have been made in understanding how microglia influence synaptic pruning process and overall brain health. Research has revealed that dysregulation in these cellular functions can contribute to the development of Alzheimer’s disease, illuminating key pathways for potential therapeutic interventions. By focusing on the role of microglia, Stevens and her team are paving the way for new treatments and diagnostic tools that could revolutionize care for millions affected by neurodegenerative conditions.
The ongoing investigation into the brain’s immune cells, often referred to as the microglia, is reshaping our understanding of various neurological disorders. These cells are integral not only in defending the brain against disease but also in maintaining the delicate balance of neural networks. Through the work of researchers like Beth Stevens, the complex interplay between microglia and neurodegenerative afflictions, including Alzheimer’s disease, is being elucidated. This research delves into how abnormal immune responses might trigger or exacerbate the progression of these conditions. As scientists continue to explore this vital area, the insights gained promise to usher in a new era of diagnostic accuracy and targeted therapeutic strategies.
Understanding Microglial Cells and Their Role in Brain Health
Microglial cells are essential components of the brain’s immune system, acting as the first line of defense against neurodegenerative diseases such as Alzheimer’s disease. These unique cells are responsible for monitoring the brain’s environment, identifying pathogens, and clearing away dead or damaged neurons. Under normal circumstances, microglia help in maintaining synaptic health by pruning unnecessary connections—essentially tidying up neuronal pathways for optimal information transmission. However, recent findings have indicated that abnormalities in this pruning process can contribute to the progression of various neurological disorders.
As highlighted in the groundbreaking research led by Beth Stevens, the improper functioning of microglial cells can exacerbate conditions like Alzheimer’s disease and Huntington’s disease. This insight has sparked a new understanding of how immune cells in the brain can influence cognitive decline and reshape our approach to treatment strategies. Engaging with fundamental questions of how these cells operate not only broadens our understanding of brain health but opens the door to innovative therapies aimed at enhancing the function of microglia, thereby mitigating the impact of neurodegenerative diseases.
Beth Stevens and the Impact of Microglial Research on Alzheimer’s Disease
Beth Stevens, a prominent neuroscientist, has devoted her career to uncovering the intricate role of microglial cells in Alzheimer’s disease. Her pioneering work has established a direct link between microglial dysfunction and the pathological features of Alzheimer’s, such as abnormal protein buildup and synaptic loss. By demonstrating how these immune cells contribute to the pruning process, Stevens has provided vital insights that could lead to new therapeutic avenues aimed at restoring normal microglial function and, ultimately, brain health.
The implications of Stevens’ research extend beyond just understanding Alzheimer’s disease; they touch on a broader spectrum of neurodegenerative diseases. Her findings point to the potential for developing new biomarkers for early detection and targeted treatments that directly address the immune functions of microglia. As researchers continue to explore the nexus between the brain’s immune system and neurodegeneration, Stevens’ contributions remain central, emphasizing that the pathway to effective therapy lies in understanding these complex biological processes.
The Importance of Foundational Research in Neurological Discoveries
Foundational research is crucial for scientific progress, especially in fields as complex as neuroscience and neurodegenerative diseases. Beth Stevens often emphasizes that breakthroughs in understanding conditions like Alzheimer’s disease can only occur through a deep-rooted commitment to basic science. By pursuing fundamental questions about how microglial cells interact with neurons and influence brain circuitry, researchers can unveil the underlying mechanisms that lead to diseases. This groundwork has the potential to inform the development of innovative treatments that can alter the course of these devastating conditions.
Moreover, the support from federal institutions like the National Institutes of Health plays an instrumental role in facilitating such research endeavors. The financial backing allows scientists to explore uncharted territories and address pressing questions about brain health and neurodegeneration. By investing in curiosity-driven science, we not only enhance our comprehension of complex biological systems but also set the stage for future advancements in the field—ultimately benefiting patients and their families struggling with challenging neurological disorders.
Funding and Support for Microglial Research Advancements
The trajectory of microglial research, particularly under the guidance of pioneers like Beth Stevens, has been significantly bolstered by robust funding from various federal agencies. This financial support enables neuroscientists to engage in exploration of innovative hypotheses regarding the role of microglia in neurodegenerative diseases such as Alzheimer’s. The resources provided allow researchers to conduct extensive studies that bridge the gap between basic science and clinical application, paving the way for the development of new treatments and diagnostic tools.
Furthermore, the acknowledgment of foundational research as a critical element in the fight against Alzheimer’s highlights the collective responsibility of the scientific community to sustain funding for such investigations. As the prevalence of Alzheimer’s continues to rise, with projections indicating that millions more will be affected in the coming decades, the urgency to understand the role of microglia becomes even more pressing. Sustaining a focus on funding microglial research can lead to breakthroughs that transform the landscape of treatment and care for millions living with these illnesses.
The Connection Between Microglial Dysfunction and Neurodegenerative Diseases
Recent advances in neuroscience have illuminated the connection between microglial dysfunction and various neurodegenerative diseases, including Alzheimer’s disease. As the brain’s immune cells, microglia are responsible for crucial tasks such as clearing debris and supporting synaptic health. However, when they become overactive or ineffective, they can contribute to the inflammatory processes that exacerbate neurodegeneration. This relationship suggests that targeting microglial activity could offer new therapeutic strategies for diseases characterized by significant cognitive decline.
Research led by scientists like Beth Stevens has revealed that abnormal microglial pruning can lead to the loss of synapses associated with cognitive function. By elucidating these mechanisms, researchers are paving the way for potential interventions that not only aim to modify the immune response but also enhance synaptic integrity in the aging brain. Understanding the nuances of microglial behavior offers hope not only for Alzheimer’s patients but for those suffering from other neurodegenerative conditions, emphasizing the need for continued investigative efforts in this domain.
Innovations in Treatment Approaches Through Microglial Research
The research into microglial function is not only providing insights into the mechanisms of neurodegenerative diseases but also catalyzing innovations in treatment approaches. By identifying the role that microglial cells play in synaptic pruning and their response to injury, scientists are beginning to develop targeted therapies that can modify the behavior of these immune cells. Such advancements could lead to the creation of drugs that improve microglial functionality, thereby offering a new avenue for combating diseases like Alzheimer’s.
Moreover, understanding microglial interactions with other brain cells paves the way for combination therapies that can tackle multiple aspects of neurodegeneration. For instance, therapies aimed at enhancing microglial activity could be used in conjunction with neuroprotective agents to better support neuron survival and synaptic health. As researchers like Beth Stevens continue to investigate these innovative treatment paradigms, the potential for meaningful breakthroughs in the management of Alzheimer’s and other neurodegenerative diseases becomes increasingly tangible.
Microglial Research and its Role in Biomarker Discovery
Microglial research is playing a pivotal role in the discovery of biomarkers for early detection of Alzheimer’s disease and other neurodegenerative conditions. By understanding how microglia respond to different pathological triggers within the brain, scientists can identify specific molecular signatures associated with disease progression. These biomarkers hold immense potential for enhancing diagnostic accuracy, enabling clinicians to detect Alzheimer’s much earlier than current methods allow.
The implications of these advancements are far-reaching. Early detection of Alzheimer’s can lead to timely interventions that may slow disease progression and improve patient outcomes. With pioneering researchers like Beth Stevens at the forefront, the integration of microglial insights into biomarker development represents a significant step forward in the fight against neurodegenerative diseases. This shift could redefine patient care and open up pathways to more personalized treatment strategies based on individual biomarker profiles.
Challenges and Future Directions in Microglial Research
While the insights garnered from microglial research are promising, numerous challenges remain in translating these findings into effective therapies for neurodegenerative diseases. One significant hurdle is the complexity of microglial dynamics within the living brain, which can vary based on numerous factors, including age, genetic background, and environmental influences. Researchers must navigate this complexity to develop interventions that can reliably target microglial function without adverse effects on brain health.
Future directions in microglial research should focus on integrating multidisciplinary approaches that combine genetics, inflammation biology, and imaging techniques to create a comprehensive understanding of microglia in neurodegenerative diseases. Collaborations between researchers, clinicians, and industry stakeholders will be essential in bridging the gap between laboratory discoveries and clinical applications. Through continued exploration and innovation in this field, scientists hope to pave the way for new strategies that will ultimately lead to better outcomes for individuals affected by Alzheimer’s disease and other cognitive disorders.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease research?
Microglial cells are crucial in Alzheimer’s disease research as they act as the brain’s immune system. They help monitor the brain for illness, remove dead cells, and prune synapses. Research led by scientists like Beth Stevens has shown that abnormal microglial pruning may contribute to the progression of Alzheimer’s and other neurodegenerative diseases.
How has Beth Stevens contributed to our understanding of microglia in neurodegenerative diseases?
Beth Stevens has significantly advanced the field of microglial research by demonstrating how these cells influence synaptic pruning and development in the brain. Her findings have unveiled the potential negative effects of microglial activity, which can play a role in neurodegenerative diseases like Alzheimer’s and Huntington’s.
What discoveries have been made about the brain immune system through microglial research?
Microglial research has led to important discoveries about the brain’s immune system, particularly its involvement in shaping synaptic connections during development. Researchers like Beth Stevens have uncovered how microglial dysfunction can lead to neurodegeneration, enhancing our understanding of diseases like Alzheimer’s.
Why is microglial research critical for developing new treatments for Alzheimer’s disease?
Microglial research is critical for Alzheimer’s treatment development as it highlights the role of microglia in disease progress. Understanding how these immune cells behave in response to pathological conditions allows researchers to design targeted therapies and establish biomarkers for earlier Alzheimer’s detection, as shown by studies from the Stevens Lab.
What implications does microglial research have for the future of neurodegenerative disease therapies?
The implications of microglial research for neurodegenerative disease therapies are profound. By uncovering the cellular mechanisms by which microglia interact with neurons, researchers can develop novel therapeutics targeting these pathways. As Beth Stevens’ work shows, this research is paving the way for potentially transformative drugs to treat Alzheimer’s disease and similar conditions.
How do microglia contribute to synaptic pruning in healthy brains versus Alzheimer’s disease?
In healthy brains, microglia actively prune synapses to refine neural circuits and promote efficient communication. However, in Alzheimer’s disease, abnormal microglial activity can lead to excessive or misguided pruning, contributing to synaptic loss and cognitive decline, as demonstrated in Beth Stevens’ research.
What funding sources support microglial research initiatives like those by Beth Stevens?
Beth Stevens’ microglial research initiatives have been significantly supported by funding from federal agencies including the National Institutes of Health (NIH). This backing is vital for pursuing innovative studies that explore the role of microglia in neurodegenerative diseases like Alzheimer’s.
What are the potential biomarkers identified in microglial research for Alzheimer’s disease detection?
Microglial research has the potential to identify biomarkers that indicate abnormal microglial activity, which could facilitate earlier detection of Alzheimer’s disease. These markers are pivotal for developing interventions before significant neurodegeneration occurs, as highlighted by findings from research at the Stevens Lab.
Why is the study of microglia important in understanding brain diseases?
Studying microglia is essential for understanding brain diseases because these immune cells play a critical role in maintaining brain health. Their involvement in synapse pruning and responses to injury or disease can reveal mechanisms underlying disorders like Alzheimer’s, leading to better prevention and treatment strategies.
How does Beth Stevens’ background inform her research on microglia and Alzheimer’s disease?
Beth Stevens’ background as a neuroscientist equipped her with the expertise to investigate complex cellular interactions in the brain, particularly the immune system’s role in brain function. Her curiosity-driven approach has allowed her to uncover the intricacies of microglial involvement in Alzheimer’s disease and other neurodegenerative conditions.
| Key Points | Details |
|---|---|
| Microglial Role | Microglia act as the immune system of the brain, monitoring health and cleaning up damaged cells. |
| Research Focus | Beth Stevens’ lab focuses on how abnormal microglial pruning could contribute to Alzheimer’s and other neurodegenerative diseases. |
| Impact of Research | The research has potential implications for developing new medications and biomarkers for Alzheimer’s disease, which affects 7 million Americans. |
| Future Projections | The incidence of Alzheimer’s cases is projected to double by 2050, increasing care costs significantly. |
| Funding Sources | The research has been largely supported by federal agencies like the National Institutes of Health, emphasizing the importance of foundational science. |
| Recognition | Beth Stevens received the MacArthur ‘genius’ award in 2015 for her contributions to microglial research. |
Summary
Microglial research has opened new avenues in understanding and combating diseases like Alzheimer’s. Beth Stevens and her laboratory have made significant breakthroughs that shed light on the dual role of microglia in brain health and disease, emphasizing the necessity of continuous investment in foundational research. As this field progresses, it holds the promise to not only alter therapeutic approaches to Alzheimer’s but also to enhance our overall comprehension of neurodegenerative disorders.